Join R4-RA
*** This trial is closed to recruitment ***
How to join R4RA as a recruiting centre
The R4-RA trial management group is keen to expand recruitment to other centres. To enquire about participation in the R4-RA trial site feasibility must be assessed.
Please contact the EMR Clinical Trials Centre to request a R4-RA Site Feasibility and Assessment Questionnaire from “Contact us” or directly contact R4RA Trial Manager:
Email: j.peel@qmul.ac.uk
Patient Information
Full information about this study is available
on the Patient Information Sheet
which should have been provided by the research doctor or nurse.
What is the purpose of this study?
RA is one of the most important inflammatory conditions in the United Kingdom and recently there have been many advances in therapy with the development of new biologic agents.
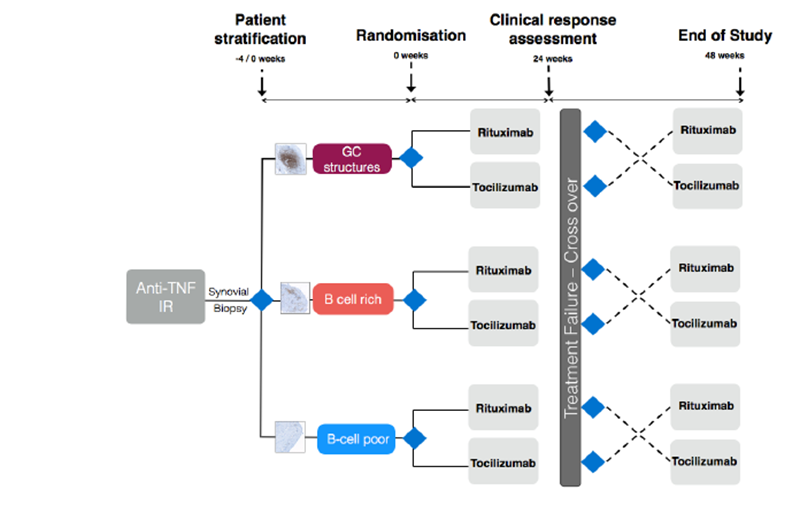
If you have been invited to be part of this study, you will have had a confirmed diagnosis of Rheumatoid Arthritis for sometime and have been tried on at least three different medications; one of which will be an anti-TNF-alpha drug. Following failure of anti-TNF therapy, there are a number of treatment options, including two drugs called Rituximab or Tocilizumab. Both treatments are licensed for use in RA at this stage in the disease.
Tocilizumab and Rituximab act in very different ways to affect the disease Ritxumab targets B cells; which are a form of white blood cell important in inflammation within the joints. Tocilizumab targets a chemical called interleukin-6 (IL-6) which mediates inflammation. At the present time, there is no clear rationale for deciding which treatment is better in patients who have failed anti-TNF therapy and therefore is very much “trial and error” - if one drug fails to work, we try another.
We have observed from our own research that patients who have B cells within the lining of inflamed joints tend to respond better to Rituximab, yet we also know that Tocilizumab is effective in patients following anti-TNF therapy.
The main purpose of this study is to try and determine if patients with no B cells in their joint lining respond better to Tocilizumab rather than Rituximab which would provide a more personalized approach to treatment decisions at this stage of their disease.
What will happen during the study?
You will attend for a screening visit to assess your eligibility for the study. There will be a full physical examination, blood tests, an ECG and urine samples. If you are eligible to take part in the study, you will undergo a biopsy of one of your joints. This involves taking a piece of tissue from your joints - please see our biopsy page. You will then be randomly assigned by a computer (neither you or the doctor decides in order to make it fair) to receive either Tocilizumab or Rituximab. We will see you monthly, with regular blood tests, ultrasound examination and x-rays before and after the study.